Chemical Formulae
Chemical formulae are symbolic representations of compounds, indicating the types and numbers of atoms present in a molecule. They are crucial for communication in chemistry and serve as a basis for understanding the stoichiometry of reactions. Let's explore some examples:
1. Water (H2O):
- Chemical Formula: \(H_2O\)
- Explanation: Water is a simple compound composed of two hydrogen atoms bonded to one oxygen atom. The subscript "2" indicates that there are two hydrogen atoms in the molecule.
2. Carbon Dioxide (CO2):
- Chemical Formula: \(CO_2\)
- Explanation: Carbon dioxide consists of one carbon atom bonded to two oxygen atoms. The subscripts "2" and "1" are implied and not usually written.
3. Sodium Chloride (NaCl):
- Chemical Formula: \(NaCl\)
- Explanation: Sodium chloride, commonly known as table salt, is an ionic compound composed of one sodium ion (\(Na^+\)) and one chloride ion (\(Cl^-\)). The formula represents the simplest ratio of sodium ions to chloride ions in the compound.
4. Ammonia (NH3):
- Chemical Formula: \(NH_3\)
- Explanation: Ammonia is a compound formed by the bonding of one nitrogen atom to three hydrogen atoms. The subscript "3" indicates that there are three hydrogen atoms in the molecule.
5. Methane (CH4):
- Chemical Formula: \(CH_4\)
- Explanation: Methane is a simple hydrocarbon consisting of one carbon atom bonded to four hydrogen atoms. The subscript "4" indicates that there are four hydrogen atoms in the molecule.
6. Sulfuric Acid (H2SO4):
- Chemical Formula: \(H_2SO_4\)
- Explanation: Sulfuric acid is a strong mineral acid composed of two hydrogen atoms, one sulfur atom, and four oxygen atoms. The subscripts "2" and "4" indicate the number of hydrogen and oxygen atoms, respectively.
7. Glucose (C6H12O6):
- Chemical Formula: \(C_6H_{12}O_6\)
- Explanation: Glucose is a simple sugar molecule consisting of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The subscripts "6" and "12" indicate the number of carbon and hydrogen atoms, respectively.
8. Carbon Tetrachloride (CCl4):
- Chemical Formula: \(CCl_4\)
- Explanation: Carbon tetrachloride is a compound composed of one carbon atom bonded to four chlorine atoms. The subscript "4" indicates that there are four chlorine atoms in the molecule.
These examples illustrate the diversity of chemical formulae and how they represent the composition of various compounds by indicating the types and numbers of atoms present in the molecule. Understanding chemical formulae is essential for communication in chemistry and for predicting the behavior of substances in chemical reactions.
Deriving the Chemical Formula of Compounds
The chemical formula of a compound is derived based on its empirical formula (the simplest whole-number ratio of atoms) and its molecular formula (the actual number of atoms of each element present). This process involves experimental analysis and mathematical calculations.
Example: Water has the empirical formula \(H_2O\), indicating that the ratio of hydrogen to oxygen atoms is 2:1. Its molecular formula is also \(H_2O\), as each water molecule consists of two hydrogen atoms bonded to one oxygen atom.
Chemical Equations
Chemical equations are concise representations of chemical reactions, showing the reactants, products, and stoichiometry of the reaction. Balancing chemical equations ensures that the law of conservation of mass is obeyed.
Balancing Chemical Equations
Balancing chemical equations involves adjusting coefficients to ensure that the number of atoms of each element is the same on both sides of the equation. This process is essential for accurately describing chemical reactions and predicting their outcomes.
Examples:
1. Combustion of Methane (CH4) with Oxygen (O2) to Produce Carbon Dioxide (CO2) and Water (H2O):
Unbalanced Equation:
\[ CH_4 + O_2 \rightarrow CO_2 + H_2O \]
Balanced Equation:
\[ CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O \]
Explanation:
To balance the equation, we add a coefficient of 2 in front of \(O_2\) to ensure there are equal numbers of oxygen atoms on both sides. This also leads to the addition of a coefficient of 2 in front of \(H_2O\) to balance the hydrogen atoms.
2. Reaction Between Hydrochloric Acid (HCl) and Sodium Hydroxide (NaOH) to Form Sodium Chloride (NaCl) and Water (H2O):
Unbalanced Equation:
\[ HCl + NaOH \rightarrow NaCl + H_2O \]
Balanced Equation:
\[ HCl + NaOH \rightarrow NaCl + H_2O \]
Explanation:
This equation is already balanced as it involves a simple acid-base neutralization reaction where one molecule of hydrochloric acid reacts with one molecule of sodium hydroxide to form one molecule of sodium chloride and one molecule of water.
3. Decomposition of Hydrogen Peroxide (H2O2) into Water (H2O) and Oxygen (O2):
Unbalanced Equation:
\[ H_2O_2 \rightarrow H_2O + O_2 \]
Balanced Equation:
\[ 2H_2O_2 \rightarrow 2H_2O + O_2 \]
Explanation:
To balance the equation, we need to add a coefficient of 2 in front of \(H_2O_2\) to ensure that there are equal numbers of hydrogen and oxygen atoms on both sides. This also leads to the formation of two water molecules on the right side of the equation.
Types of State Symbols
State symbols indicate the physical states of substances involved in chemical reactions. Common symbols include \(s\) for solid, \(l\) for liquid, \(g\) for gas, and \(aq\) for aqueous (dissolved in water). These symbols provide crucial information about the conditions under which reactions occur.
More on AmplifyGlobe
Ion Formation
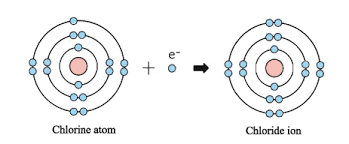 Ion formation is a foundational process in chemistry that profoundly influences Read More
Ion formation is a foundational process in chemistry that profoundly influences Read More
If you're looking to ace your ATI TEAS test and get accepted into the nursing program of your dreams, try ExamGates today. Tutors who have taken the exam before wrote and prepared the practice questions on ExamGates. Therefore, you have 100% relevant content, vivid images and illustrations, and in-depth explanations for right and wrong answers.