In the following single-replacement reaction, ______ replaces ______. Cl2+2NaI→2NaCl+I2
A. sodium, iodine
B. chlorine, iodine
C. chlorine, sodium
D. sodium, chlorine
For those aiming to excel in their ATI TEAS test and secure admission into their desired nursing program, ExamGates offers an invaluable resource. Our platform features practice questions meticulously crafted by tutors who have previously aced the exam themselves. With ExamGates, you can access content that is 100% relevant to the test, accompanied by vivid images and illustrations. Additionally, our platform provides comprehensive explanations for both correct and incorrect answers, empowering you to fully grasp the material and optimize your study efforts. Take the first step towards your nursing aspirations with ExamGates today.
In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
Therefore, the Correct Answer is B.
More Questions on TEAS 7 Science Practice Test 2
Question 1:
Blood oxygen levels are most likely low when blood _____.
A. leaves the aorta
B. fills the right atrium
C. reaches body tissues
D. flows through arteries
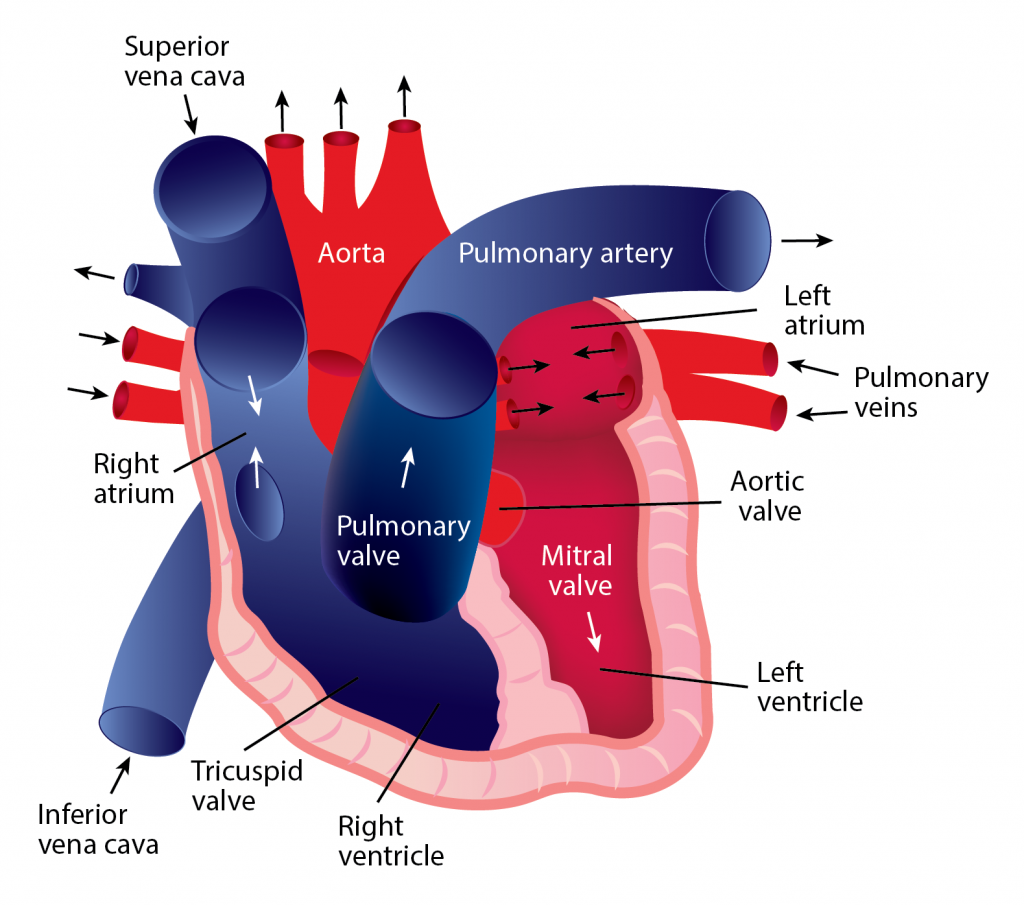
The Correct Answer is B.Blood continually flows in one direction, beginning in the heart and proceeding to the arteries, arterioles, and capillaries. When blood reaches the capillaries, exchanges occur between blood and tissues. After this exchange happens, blood is collected into venules, which feed into veins and eventually flow back to the heart’s atrium. The heart must relax between two heartbeats for blood circulation to begin.
Two types of circulatory processes occur in the body:
Systemic circulation
- The pulmonary vein pushes oxygenated blood into the left atrium.
- As the atrium relaxes, oxygenated blood drains into the left ventricle through the mitral valve. 3. The left ventricle pumps oxygenated blood to the aorta.
- Blood travels through the arteries and arterioles before reaching the capillaries that surround the tissues.
Pulmonary circulation
- Two major veins, the Superior Vena Cava and the Inferior Vena Cava, brings deoxygenated blood from the upper and lower half of the body.
- Deoxygenated blood is pooled into the right atrium and then sent into the right ventricle through the tricuspid valve, which prevents blood from flowing backward.
- The right ventricle contracts, causing the blood to be pushed through the pulmonary valve into the pulmonary artery.
- Deoxygenated blood becomes oxygenated in the lungs.
- Oxygenated blood returns from the lungs to the left atrium through the pulmonary veins.
Question 2:
When would a cell most likely contain the most nucleotides?
A. S
B. G1
C. M
D. G2
The Correct Answer is B.A cell copies its DNA during the S phase, and nucleotides are the building blocks of DNA. Thus, the step preceding the S phase, the G1 phase, is the phase of the cell cycle when the cell would contain the most nucleotides.
For a cell to divide into more cells, it must grow, copy its DNA, and produce new daughter cells. The cell cycle regulates cellular division. This process can either prevent a cell from dividing or trigger it to start dividing.
The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
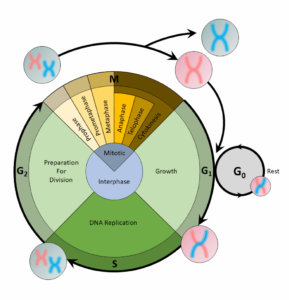
- G1: The first gap phase, during which the cell prepares to copy its DNA
- S: The synthesis phase, during which DNA is copied
- G2 : The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotides for synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
Question 3:
In which state of matter do the particles of iron have the lowest amount of cohesion?
A. Solid iron particles have the lowest amount of cohesion
B. Liquid iron particles have the lowest amount of cohesion
C. Gaseous iron particles have the lowest amount of cohesion
D. The particles have the same amount of cohesion in all states of matter.
The Correct Answer is C.The particles in a sample of gas are farther apart than in solids or liquids and therefore have the lowest amount of cohesion.
- Cohesion is the tendency of particles of the same kind to stick to each other.
- A solid has the lowest amount of energy because its particles are packed close together. Liquids have more energy than a solid, and gases have more energy than solids or liquids because the cohesive forces are very weak.
Question 4:
The diffusion of nutrients through the walls of the digestive system is critical to homeostasis in the body. Where does the majority of this diffusion take place in the digestive system?
A. Stomach
B. Esophagus
C. Oral cavity
D. Small intestine
The Correct Answer is D.The duodenum is the first part of the small intestines, located between the stomach and the middle part of the small intestines (jejunum). Once food has mixed with acid in the stomach, it moves into the duodenum, where it then mixes with bile from the gallbladder and digestive juices secreted from the pancreas. In the duodenum, absorption of vitamins, minerals, and nutrients begins.
Question 5:
What type of reaction is described by the following equation?
ZnBr2(aq) + 2KOH(aq) → Zn(OH)2(s) + 2KBr(aq)
A. Synthesis
B. Decomposition
C. Single-Replacement
D. Double-Replacement
The Correct Answer is D.In this reaction, two elements are trading places hence double-replacement. In the reactants, zinc and bromide ions are together, and potassium and hydroxide ions are together. In the products, zinc and hydroxide ions are together, and potassium and bromide ions are together.
Question 6:
_____ is dependent not only on the temperature, but also on the amount of substance available.
A. Condensation
B. Deposition
C. Evaporation
D. Melting
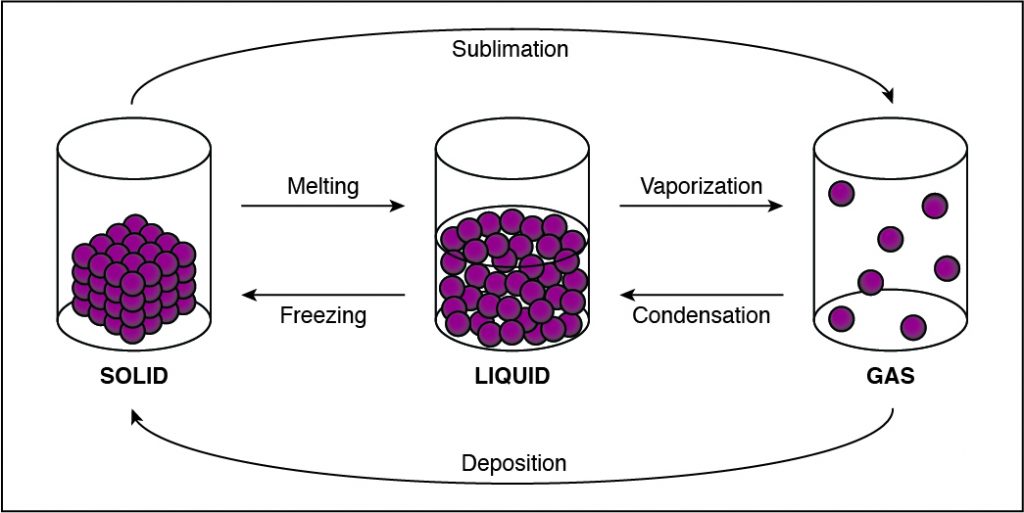
The Correct Answer is C.Unlike condensation, deposition, and melting, evaporation is dependent not only on the temperature, but also on the amount of a substance available.
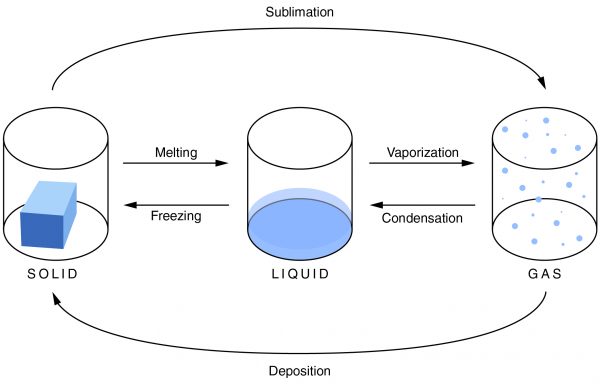
Condensation is the change of a gas or vapor to a liquid. A change in the pressure and the temperature of a substance causes this change. The condensation point is the same as the boiling point of a substance. It is most noticeable when there is a large temperature difference between an object and the atmosphere. Condensation is also the opposite of evaporation.
Evaporation is the change of a liquid to a gas on the surface of a substance. This is not to be confused with boiling, which is a phase transition of an entire substance from a liquid to a gas. The evaporation point is the same as the freezing point of a substance. As the temperature increases, the rate of evaporation also increases. Evaporation depends not only on the temperature, but also on the amount of substance available.
Freezing is the change of a liquid to a solid. It occurs when the temperature drops below the freezing point. The amount of heat that has been removed from the substance allows the particles of the substance to draw closer together, and the material changes from a liquid to a solid. It is the opposite of melting.
Melting is the change of a solid into a liquid. For melting to occur, enough heat must be added to the substance. When this is done, the molecules move around more, and the particles are unable to hold together as tightly as they can in a solid. They break apart, and the solid becomes a liquid.
Sublimation is a solid changing into a gas. As a material sublimates, it does not pass through the liquid state. An example of sublimation is carbon dioxide, a gas, changing into dry ice, a solid. It is the reverse of deposition.
Deposition is a gas changing into a solid without going through the liquid phase. It is an uncommon phase change. An example is when it is extremely cold outside and the cold air comes in contact with a window. Ice will form on the window without going through the liquid state.
Question 7:
What solution has a pH of 7?
A. Aniline
B. Pyridine
C. Pure water
D. Sodium hydroxide
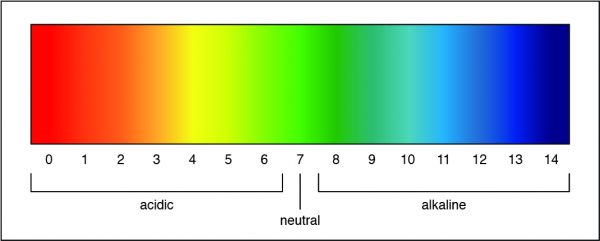
The Correct Answer is C.A pH of 7 is a neutral solution, which is how pure water is classified. Researchers can determine the strength of an acid or a base by measuring the pH of a solution. The pH value describes how acidic or basic a solution is. On pH scale, shown below, if the number is less than 7 the solution is acidic. A pH greater than 7 means the solution is basic. When the pH is exactly 7, the solution is neutral.
Question 8:
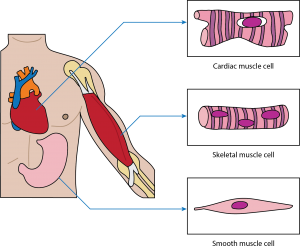
Where is skeletal muscle found?
A. Inside the heart
B. Attached to bone
C. Lining the walls of the bladder
D. Within the gastrointestinal tract
The Correct Answer is B.Skeletal muscle: This muscle cell is striated, long, and cylindrical. There are many nuclei in a skeletal muscle cell. Attached to bones in the body, skeletal muscle contracts voluntarily, meaning that it is under conscious control.
Smooth muscle: This muscle consists of nonstriated muscle cells that are spindle-shaped. Like cardiac muscle cells, smooth muscle cells contain one nucleus. This muscle type is found in the walls of internal organs like the bladder and stomach. Smooth muscle contraction is involuntary and controlled by the autonomic nervous system.
Cardiac muscle: This muscle consists of muscle cells that are striated, short, and branched. These cells contain one nucleus, are branched, and are rectangular. Cardiac muscle contraction is an involuntary process, which is why it is under the control of the autonomic nervous system. This muscle is found in the walls of the heart.
Question 9:
During which of the following phase changes will the cohesion between the particles in a substance decrease?
A. Condensation
B. Deposition
C. Freezing
D. Vaporization
The Correct Answer is D.If the cohesion between particles decreases, then the particles must be undergoing a phase change that allows particles to move farther apart. This happens when a substance vaporizes and turns from liquid to gas. Any phase change that moves to the right in the diagram above requires energy to be added to the system because the substance has more energy at the end of the phase change. The phase changes are melting, vaporization (boiling), and sublimation. When energy is added, particles move faster and can break away from each other more easily as they move to a state of matter with a higher amount of energy. This is most commonly done by heating the substance.
Question 10:
What body system is the skeletal system most closely associated with when hematopoiesis happens?
A. Urinary system
B. Digestive system
C. Muscular system
D. Cardiovascular system
The Correct Answer is D.The cardiovascular system is closely associated with hematopoiesis because it includes the heart and blood vessels, which are responsible for circulating blood throughout the body. Hematopoiesis, the process of blood cell formation, primarily occurs in the bone marrow, which is part of the skeletal system. However, the cardiovascular system plays a crucial role in transporting these blood cells to various parts of the body once they are produced in the bone marrow.
So, while the skeletal system provides the site for hematopoiesis, the cardiovascular system is responsible for distributing the blood cells, making it the most closely associated system in this context.