Using the equation PH = -log (H+), a solution with a (H+)= 10+ M has a PH of which of the following?
A. 9
B. 5
C. 1
D. 10
For those aiming to excel in their ATI TEAS test and secure admission into their desired nursing program, ExamGates offers an invaluable resource. Our platform features practice questions meticulously crafted by tutors who have previously aced the exam themselves. With ExamGates, you can access content that is 100% relevant to the test, accompanied by vivid images and illustrations. Additionally, our platform provides comprehensive explanations for both correct and incorrect answers, empowering you to fully grasp the material and optimize your study efforts. Take the first step towards your nursing aspirations with ExamGates today.
Concentration of H+ in the solution = 10 −1 M
pH= -log(10 −1)
pH= -(-1)
=1
Therefore, the Correct Answer is C.
More Questions on TEAS 7 Science Exam 5
Question 1:
Escherichia cell is plated on nutrient agar plates that each contain a different type of antibiotic. The shaded area represents growth of the bacteria. Which of the following plates contains bacteria that were most resistant to the antibiotic
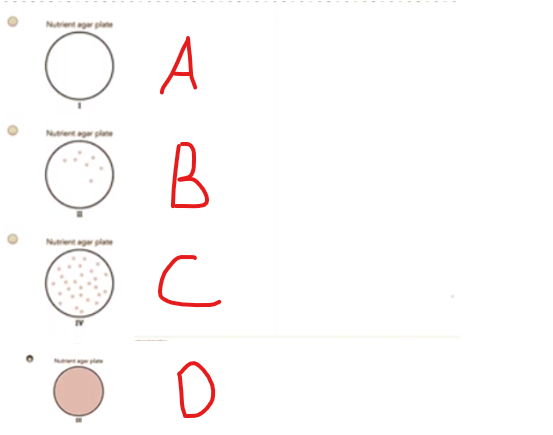
A. A
B. B
C. C
D. D
The Correct Answer is C.This is the plate with the most growth despite the presence of antibiotic cover.
Question 2:
Which of the following types of cells stimulates other immune cells to attack and destroy foreign agents?
A. Cytotoxic T-cells
B. Plasma cells
C. Natural killer cells
D. Helper T-Cells
The Correct Answer is D.Helper T cells are important cells that stimulate the action of other immune cells. For instance, they not only help activate B cells to secrete antibodies and macrophages to destroy ingested microbes, but they also help activate cytotoxic T cells to kill infected target cells.
Question 3:
Which of the following tools can be used to measure the turbidity of a liquid by measuring the transmission of light through the sample?
A. Spectrophotometer
B. Centrifuge
C. Electro photometer
D. Microdensitometer
The Correct Answer is A.A spectrophotometer is an instrument that measures the intensity of light absorbed after it passes through a sample solution. With the spectrophotometer, the concentration of a chemical substance can be determined by measuring the intensity of light detected.
Question 4:
Which of the following characteristics of water helps explain why coastal areas experience less dramatic temperature changes during the day?
A. Water has a high specific heat capacity
B. Water is a versatile solvent
C. Water forms covalent bonds with other water molecules
D. Water's adhesive properties prevent evaporation
The Correct Answer is A.Water has a high specific heat capacity, which means it can absorb and store a large amount of heat energy without significantly changing in temperature. This helps moderate the temperature fluctuations of the air near the coast, as water can store heat during the day and release it at night, or vice versa.
Question 5:
A strand of DNA bases reads 5' AGCTAGCGT 3, what would the sequence of bases on the contemporary strand read?
A. 3' STOGUTCGCU
B. 5' AGCTAGCGT S
C. 3' SUCGAUCICA
D. 3' TCGATCGCA 5'
The Correct Answer is D.The complementary strand of DNA is formed by pairing complementary bases. In DNA, adenine (A) pairs with thymine (T), and guanine (G) pairs with cytosine (C). Additionally, RNA uses uracil (U) instead of thymine.
Given the sequence 5' AGCTAGCGT 3', the complementary strand would read:
3' TCGATCGCA 5'
Question 6:
Which of the following is classified as a noninfectious disease?
A. Psoriasis
B. Brucellosis
C. Olkunginys
D. Dengue
The Correct Answer is A.Psoriasis is a chronic skin condition that causes red, scaly patches on the skin. It is not contagious, and it is not caused by any microorganism. It is believed to be an autoimmune disorder where the immune system attacks healthy skin cells.
Question 7:
Which of the following is correct regarding the pH scale?
A. A substance with a pH of 3 is two times more alkaline than a substance with a pH of 4
B. A substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4
C. A substance with a pH of 3 is two times more acidic than a substance with a pH of 4
D. A substance with pH of 3 is 10 times more alkaline than a substance with a pH of 4
The Correct Answer is B.The pH scale is logarithmic, indicating that each whole number change on the pH scale represents a tenfold change in acidity or alkalinity. Therefore, a substance with a pH of 3 is 10 times more acidic than a substance with a pH of 4.
Question 8:
A slice of apple left on a table slowly goes brown due to an enzymatic reaction. Dipping the apple slice in lemon juice prevents it from browning Which of the following best explains this result?
A. Lemon juice has enzymes which reverse the browning reaction
B. Lemon juice has a pH which inactivates the enzymes
C. Lemon juice functions to bleach the brown material
D. Lemon juice functions to dilute the brown material
The Correct Answer is B.Browning of the apple surface is due to the action of the enzyme polyphenol oxidase. Lemon juice is acidic and lowers the pH of the apple's surface. This inactivates the enzyme polyphenol oxidase, which works best at a neutral pH. This in turn reduces the browning action on the apple.
Question 9:
Which of the following structures drains oxygen-depleted blood from the kidneys?
A. Renal artery
B. Urethra
C. Ureter
D. Renal Vein
The Correct Answer is D.The renal vein drains deoxygenated blood from the kidneys to the inferior vena cava.
Question 10:
Which of the following molecules contains the fewest covalent bonds?
A. Chlorine molecule
B. Ammonia molecule
C. Methane molecule
D. Water molecule
The Correct Answer is A.A chlorine molecule contains the fewest covalent bonds among the options provided. It consists of two chlorine atoms bonded together by a single covalent bond. Compared with the other options, ammonia has three covalent bonds, methane has 4 while water has 2.