What type of bond forms between nitrogen and oxygen, and why?
A. Ionic, because electrons are shared
B. Covalent, because electrons are shared
C. Ionic, because electrons are transferred
D. Covalent, because electrons are transferred
For those aiming to excel in their ATI TEAS test and secure admission into their desired nursing program, ExamGates offers an invaluable resource. Our platform features practice questions meticulously crafted by tutors who have previously aced the exam themselves. With ExamGates, you can access content that is 100% relevant to the test, accompanied by vivid images and illustrations. Additionally, our platform provides comprehensive explanations for both correct and incorrect answers, empowering you to fully grasp the material and optimize your study efforts. Take the first step towards your nursing aspirations with ExamGates today.
Nitrogen and oxygen are both nonmetals, which means they will share electrons in a covalent bond. For example, two oxygen atoms form a double bond, in which two pairs of electrons (four electrons total) are shared. Similarly, two nitrogen atoms form a molecule with a triple bond, in which three pairs of electrons (six electrons total) are shared.
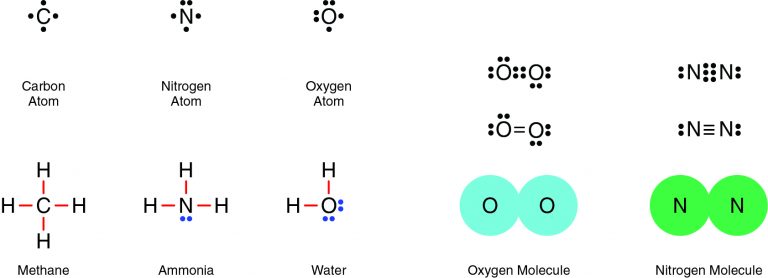
Therefore, the Correct Answer is B.
More Questions on TEAS 7 Science Practice Test 2
Question 1: A person is diagnosed as having acidosis, a condition in which the blood pH is below 7.45. What does the doctor most likely conclude?
A. Too much carbon dioxide is found in the blood.
B. Highly oxygenated blood circulates through the body
C. A blockage prevents blood from leaving the pulmonary artery
D. The nasal cavity has a difficult time clearing particles from the air.
The Correct Answer is A. Acidosis is when the body fluids contain too much acid, or low pH. The kidneys and lungs are unable to keep the body’s pH in balance. Acidosis is the result when there is too much loss of bicarbonate from the blood known as metabolic acidosis, or due to a buildup of carbon dioxide in the blood due to poor lung function, known as respiratory acidosis. It is the opposite of alkalosis, which is a condition in which there is too much base in the body fluids.Question 2:
Which is classified as a type of acid-base reaction that produces a salt?
A. Combination
B. Decomposition
C. Hydrolysis
D. Neutralization
The Correct Answer is D.A neutralization reaction is a type of acid-base reaction where an acid and base react to form a salt and water.
In an aqueous solution, a base increases the hydroxide concentration (OH–), while an acid increases the hydrogen ion (H+) concentration. Sometimes, neutralization reactions also occur. This type of reaction happens when an acid and a base react with each other to form water and salt. Salt is typically defined as an ionic compound that includes any cation except H+ and any anion except OH–. Consider the following example of a neutralization reaction between hydrobromic acid (HBr) and potassium hydroxide (KOH).
HBr+KOH→KBr+H2O
Not all neutralization reactions proceed in the manner where all reactants are in the aqueous phase. In some chemical reactions, one reactant may be a solid. The neutralization reaction can still proceed to completion.
Question 3:
Which of the following is a component of a chromosome?
A. Centromere
B. Gamete
C. Homologue
D. Ribose
The Correct Answer is A.The protein disc that holds two sister chromatids together is what collectively makes a chromosome. A gene is a segment of DNA, deoxyribonucleic acid, which transmits information from parent to offspring. A single molecule of DNA has thousands of genes. A chromosome is a rod-shaped structure that forms when a single DNA molecule and its associated proteins coil tightly before cell division.
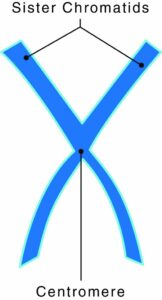
Chromosomes have two components:
- Chromatids: two copies of each chromosome
- Centromeres: protein discs that attach the chromatids together
Human cells have 23 sets of different chromosomes. The two copies of each chromosome are called homologous chromosomes, or homologues. An offspring receives one homologue from each parent. When a cell contains two homologues of each chromosome, it is termed diploid (2n). A haploid (n) cell contains only one homologue of each chromosome. The only haploid cells humans have are the sperm and eggs cells known as gametes.
Question 4:
In the following single-replacement reaction, ______ replaces ______.
Cl2+2NaI→2NaCl+I2
A. sodium, iodine
B. chlorine, iodine
C. chlorine, sodium
D. sodium, chlorine
The Correct Answer is B.In this reaction, chlorine (Cl2) is an element in the reaction that replaces iodine in the compound sodium iodide (NaI). This allows chlorine to form a compound with sodium (NaCl) and leaves iodine (I2) as an element.
Synthesis reactions involve two or more reactants (A and B) combining to form one product (AB). In the example provided, hydrogen (H2) and oxygen (O2) begin as separate elements. At the end of the reaction, the hydrogen and oxygen atoms are bonded in a molecule of water (H2O).
Decomposition reactions have only one reactant (AB) that breaks apart into two or more products (A and B). In the example above, hydrogen peroxide (H2O2) breaks apart into two smaller molecules: water (H2O) and oxygen (O2).
Single-replacement reactions involve two reactants, one compound (AB) and one element (C). In this type of reaction, one element replaces another to form a new compound (AC), leaving one element by itself (B). In the example, zinc replaces hydrogen in hydrochloric acid (HCl). As a result, zinc forms a compound with chlorine, zinc chloride (ZnCl2), and hydrogen (H2) is left by itself.
Double-replacement reactions involve two reactants, both of which are compounds made of two components (AB and CD). In the example, silver nitrate, composed of silver (Ag1+) and nitrate (NO31-) ions, reacts with sodium chloride, composed of sodium (Na1+) and chloride (Cl1-) ions. The nitrate and chloride ions switch places to produce two compounds that are different from those in the reactants.
Combustion reactions occur when fuels burn, and they involve specific reactants and products, as seen in the examples below. Some form of fuel that contains carbon and hydrogen is required. Examples of such fuels are methane, propane in a gas grill, butane in a lighter, and octane in gasoline. Notice that these fuels all react with oxygen, which is necessary for anything to burn. In all combustion reactions, carbon dioxide, water, and energy are produced. When something burns, energy is released, which can be felt as heat and seen as light.
Question 5:
When would a cell most likely contain the most nucleotides?
A. S
B. G1
C. M
D. G2
The Correct Answer is B.A cell copies its DNA during the S phase, and nucleotides are the building blocks of DNA. Thus, the step preceding the S phase, the G1 phase, is the phase of the cell cycle when the cell would contain the most nucleotides.
For a cell to divide into more cells, it must grow, copy its DNA, and produce new daughter cells. The cell cycle regulates cellular division. This process can either prevent a cell from dividing or trigger it to start dividing.
The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
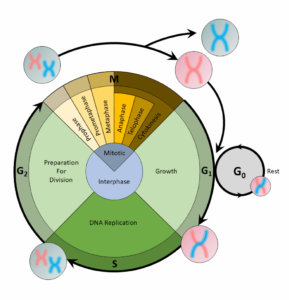
- G1: The first gap phase, during which the cell prepares to copy its DNA
- S: The synthesis phase, during which DNA is copied
- G2 : The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotides for synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
Question 6:
What is the correct order of the stages of the cell cycle?
A. G1,S,G2,M
B. G2,S,G1,M
C. M,S,G2,G1
D. S,M,G1,G1
The Correct Answer is A.The cell cycle is an organized process divided into two phases: interphase and the M (mitotic) phase. During interphase, the cell grows and copies its DNA. After the cell reaches the M phase, division of the two new cells can occur. The G1, S, and G2 phases make up interphase.
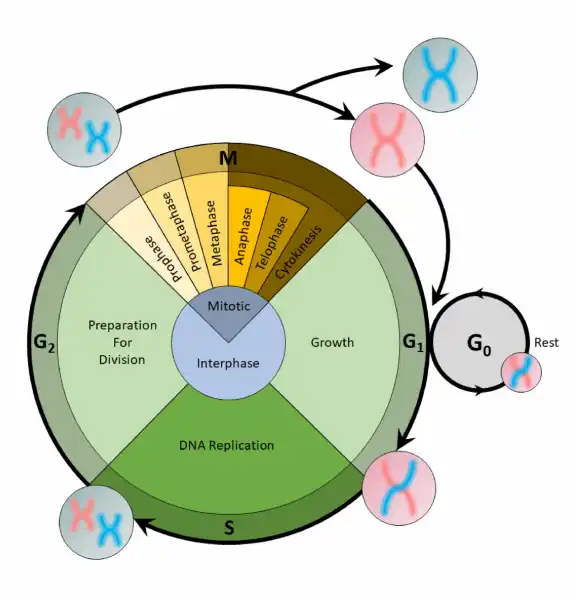
- G1: The first gap phase, during which the cell prepares to copy its DNA
- S: The synthesis phase, during which DNA is copied
- G2 : The second gap phase, during which the cell prepares for cell division
It may appear that little is happening in the cell during the gap phases. Most of the activity occurs at the level of enzymes and macromolecules. The cell produces things like nucleotides for synthesizing new DNA strands, enzymes for copying the DNA, and tubulin proteins for building the mitotic spindle. During the S phase, the DNA in the cell doubles, but few other signs are obvious under the microscope. All the dramatic events that can be seen under a microscope occur during the M phase: the chromosomes move, and the cell splits into two new cells with identical nuclei.
Question 7:
After food has been masticated in the oral cavity, where does it go next?
A. Colon
B. Liver
C. Pancreas
D. Pharynx
The Correct Answer is D.Once the food has been masticated in the oral cavity (mouth), it is then swallowed and travels back into the pharynx down into the esophagus, which leads into the stomach.
Question 8:
The diffusion of nutrients through the walls of the digestive system is critical to homeostasis in the body. Where does the majority of this diffusion take place in the digestive system?
A. Stomach
B. Esophagus
C. Oral cavity
D. Small intestine
The Correct Answer is D.The duodenum is the first part of the small intestines, located between the stomach and the middle part of the small intestines (jejunum). Once food has mixed with acid in the stomach, it moves into the duodenum, where it then mixes with bile from the gallbladder and digestive juices secreted from the pancreas. In the duodenum, absorption of vitamins, minerals, and nutrients begins.
Question 9:
What raw inorganic material would an autotroph most likely use to create chemical energy for growth?
A. carbon dioxide
B. minerals in soil
C. decaying matter
D. sugar molecules
The Correct Answer is B.Autotrophs are organisms that use basic raw materials in nature, like the sun, to make energy-rich biomolecules. Minerals are naturally inorganic.
Autotrophs are organisms that make energy-rich biomolecules from raw material in nature. They do this by using basic energy sources such the sun. This explains why most autotrophs rely on photosynthesis to transform sunlight into usable food that can produce energy necessary for life. Plants and certain species of bacteria are autotrophs.
Question 10:
_____ is dependent not only on the temperature, but also on the amount of substance available.
A. Condensation
B. Deposition
C. Evaporation
D. Melting
The Correct Answer is C.Unlike condensation, deposition, and melting, evaporation is dependent not only on the temperature, but also on the amount of a substance available.
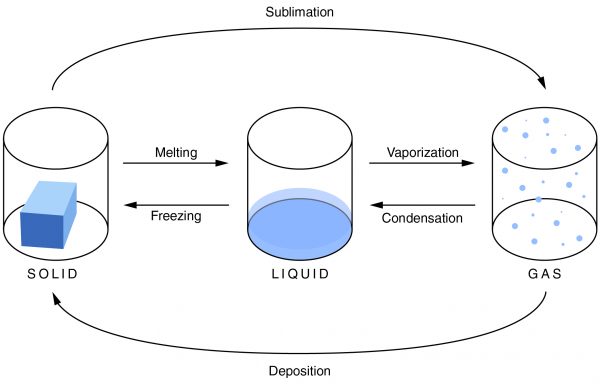
Condensation is the change of a gas or vapor to a liquid. A change in the pressure and the temperature of a substance causes this change. The condensation point is the same as the boiling point of a substance. It is most noticeable when there is a large temperature difference between an object and the atmosphere. Condensation is also the opposite of evaporation.
Evaporation is the change of a liquid to a gas on the surface of a substance. This is not to be confused with boiling, which is a phase transition of an entire substance from a liquid to a gas. The evaporation point is the same as the freezing point of a substance. As the temperature increases, the rate of evaporation also increases. Evaporation depends not only on the temperature, but also on the amount of substance available.
Freezing is the change of a liquid to a solid. It occurs when the temperature drops below the freezing point. The amount of heat that has been removed from the substance allows the particles of the substance to draw closer together, and the material changes from a liquid to a solid. It is the opposite of melting.
Melting is the change of a solid into a liquid. For melting to occur, enough heat must be added to the substance. When this is done, the molecules move around more, and the particles are unable to hold together as tightly as they can in a solid. They break apart, and the solid becomes a liquid.
Sublimation is a solid changing into a gas. As a material sublimates, it does not pass through the liquid state. An example of sublimation is carbon dioxide, a gas, changing into dry ice, a solid. It is the reverse of deposition.
Deposition is a gas changing into a solid without going through the liquid phase. It is an uncommon phase change. An example is when it is extremely cold outside and the cold air comes in contact with a window. Ice will form on the window without going through the liquid state.