Which of the following microorganisms lack their own metabolic pathways and can only reproduce inside of a host cell?
A. Bacteria
B. Protozoa
C. Helminths
D. Viruses
For those aiming to excel in their ATI TEAS test and secure admission into their desired nursing program, ExamGates offers an invaluable resource. Our platform features practice questions meticulously crafted by tutors who have previously aced the exam themselves. With ExamGates, you can access content that is 100% relevant to the test, accompanied by vivid images and illustrations. Additionally, our platform provides comprehensive explanations for both correct and incorrect answers, empowering you to fully grasp the material and optimize your study efforts. Take the first step towards your nursing aspirations with ExamGates today.
Viruses.
Viruses lack essential machinery needed to reproduce by themselves.
In fact, viruses can only reproduce after infecting a living cell - a process called viral replication.
Once inside a living cell, viruses re-program the cell’s machinery to produce viral proteins and genetic material to make new copies of themselves.
Choice A, Bacteria, is not the correct answer because bacteria have their own metabolic pathways and can reproduce outside of a host cell.
Choice B, Protozoa, is also not the correct answer because protozoa are singlecelled eukaryotes that have their own metabolic pathways and can reproduce outside of a host cell.
Choice C, Helminths, is not the correct answer because helminths are multicellular parasitic worms that have their own metabolic pathways and can reproduce outside of a host cell.
Therefore, the Correct Answer is D.
More Questions on TEAS 7 Science Exam 2
Question 1:
Nitrogen gas is an extremely stable molecule because of which of the following?
A. Ionic bonds
B. Hydrogen bonds
C. Resonance bonds
D. Triple covalent bonds
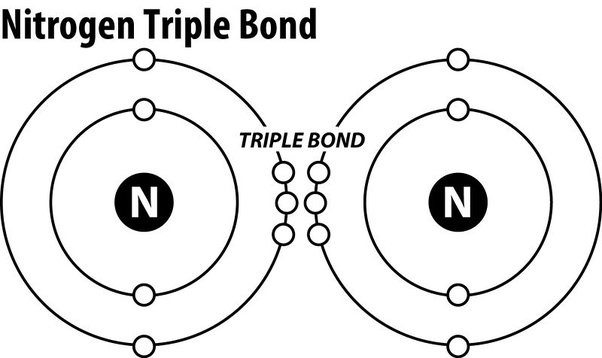
The Correct Answer is D.Triple covalent bonds.
Nitrogen gas (N2) is an extremely stable molecule because it consists of two nitrogen atoms bonded together by a triple covalent bond.
A covalent bond is a type of chemical bond where atoms share electrons to form a molecule.
In a triple covalent bond, three pairs of electrons are shared between the two atoms, resulting in a very strong bond that makes the molecule extremely stable.
Choice A.
Ionic bonds is not correct because ionic bonds involve the transfer of electrons from one atom to another to form ions, which are then attracted to each other due to their opposite charges.
Nitrogen gas does not contain ions and is not held together by ionic bonds.
Choice B.
Hydrogen bonds is not correct because hydrogen bonds are weak electrostatic attractions between molecules that contain hydrogen atoms bonded to highly electronegative atoms such as oxygen or nitrogen.
Nitrogen gas does not contain hydrogen atoms and is not held together by hydrogen bonds.
Choice C.
Resonance bonds is not correct because resonance refers to the delocalization of electrons in a molecule where multiple Lewis structures can be drawn to represent the molecule.
Nitrogen gas has a single Lewis structure and does not exhibit resonance.
Question 2:
To separate genomic DNA fragments by size, which of these laboratory methods is most useful?
A. Titration
B. Electrophoresis
C. Filtration
D. Spectrophotometry
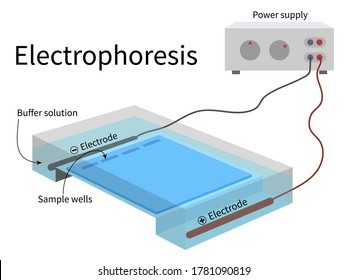
The Correct Answer is B.Electrophoresis is the most useful laboratory method for separating genomic DNA fragments by size.
Electrophoresis is a technique that uses an electric field to separate charged molecules, such as DNA fragments, based on their size and charge.
Choice A is not correct because titration is a laboratory method used to determine the concentration of a solution.
Choice C is not correct because filtration is a laboratory method used to separate solids from liquids.
Choice D is not correct because spectrophotometry is a laboratory method used to measure the absorbance of light by a solution.
Question 3:
Which of the following indicates the function of sodium bicarbonate secreted by the pancreas?
A. Sodium bicarbonate is a protease that digests carbohydrates.
B. Sodium bicarbonate stimulates the pyloric sphincter.
C. Sodium bicarbonate inhibits peristalsis.
D. Sodium bicarbonate neutralizes the acidity of chyme.
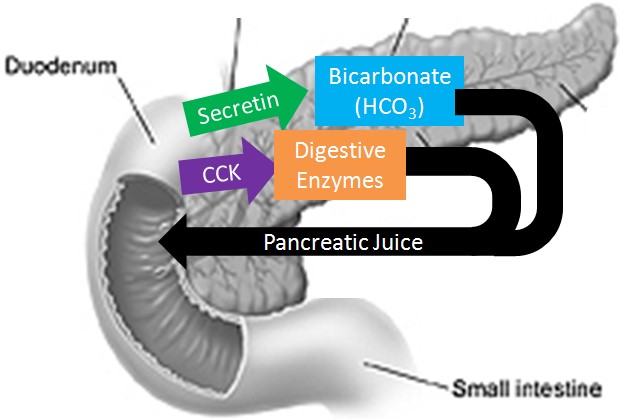
The Correct Answer is D.Sodium bicarbonate neutralizes the acidity of chyme.
The pancreas secretes large amounts of sodium bicarbonate, which protects the duodenum by neutralizing the acid that comes from the stomach.
This compound helps neutralize stomach acid generated during the digestive process.
Choice A is incorrect because sodium bicarbonate is not a protease that digests carbohydrates.
Proteases are enzymes that break down proteins, while sodium bicarbonate is a chemical compound that helps neutralize stomach acid.
Choice B is incorrect because sodium bicarbonate does not stimulate the pyloric sphincter.
The pyloric sphincter is a ring of smooth muscle that separates the stomach from the duodenum and regulates the passage of partially digested food (chyme) into the small intestine.
Choice C is incorrect because sodium bicarbonate does not inhibit peristalsis.
Peristalsis is a series of wave-like muscle contractions that move food through the digestive tract.
Question 4:
Which of the following is a group that can be measured against the experimental group?
A. Responding
B. Manipulated
C. Control
D. Variable
The Correct Answer is C.Control.
A control group is a group in an experiment that does not receive the treatment or manipulation being tested and is used as a benchmark to measure how the other tested subjects do.
The control group is used to minimize the effects of all variables except the independent variable.
This allows researchers to determine if changes in the dependent variable are due to the manipulation of the independent variable or if they are due to some other factor.
Choice A.
Responding is not the correct answer because it refers to the dependent variable, which is the variable that is being measured in an experiment.
Choice B.
Manipulated is not the correct answer because it refers to the independent variable, which is the variable that is being manipulated in an experiment.
Choice D.
Variable is not the correct answer because it refers to any factor that can change in an experiment and can include both independent and dependent variables.
Question 5:
In a phase diagram, which of the following is the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously?
A. Triple point
B. Critical temperature
C. Critical point
D. Absolute zero
The Correct Answer is A.Triple point.
In a phase diagram, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously is the triple point.
The triple point is a unique point on a phase diagram where the three states of matter (solid, liquid, and gas) can coexist in equilibrium.
At the triple point, the temperature and pressure of the substance are fixed.
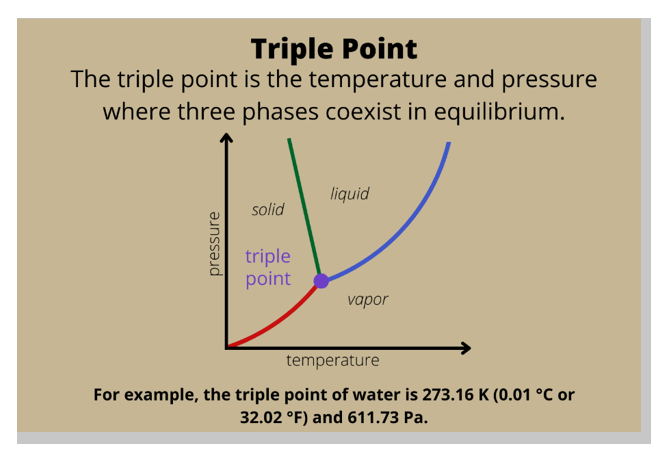
Option B, critical temperature, is the temperature at which a gas cannot be liquefied, regardless of the pressure applied.
It is a characteristic property of a substance and is typically higher than the boiling point of the liquid at standard pressure.
Option C, critical point, is the point on a phase diagram where the liquid and gas phases of a substance become indistinguishable.
At the critical point, the distinction between the liquid and gas phases disappears, and the substance becomes a supercritical fluid.
Option D, absolute zero, is the theoretical temperature at which all matter has zero thermal energy.
At absolute zero, all substances are in their solid state, but it is not relevant to a phase diagram, as it is a temperature where no transitions between states occur.
In summary, the term used for a substance held at a temperature and pressure where the solid, liquid, and gaseous states of a substance exist simultaneously in a phase diagram is the triple point, whereas the other options provided are not relevant or are characteristic properties of substances in different contexts.
Question 6:
Which of the following is the number of protons in a lithium atom?
A. 7
B. 3
C. 12
D. 4
The Correct Answer is B.The atomic number of an element represents the number of protons in the nucleus of an atom of that element.
Since lithium has an atomic number of 3, it has 3 protons in its nucleus.
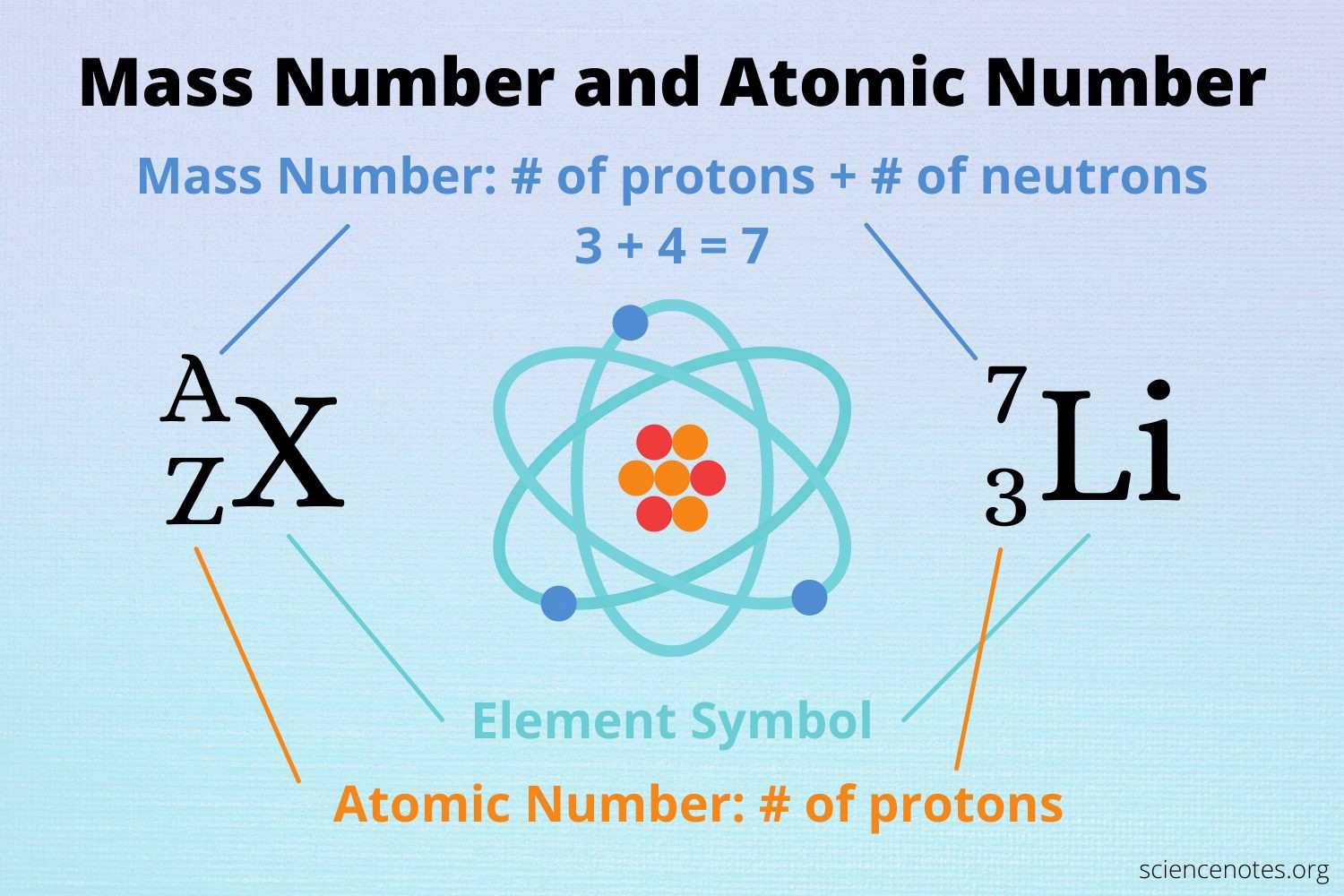
Choice A is not correct because 7 is the mass number of lithium, not the number of protons.
Choice C is not correct because 12 is not the atomic number or mass number of lithium.
Choice D is not correct because 4 is not the atomic number or mass number of lithium.
Question 7:
Which of the following glands synthesizes antidiuretic hormone?
A. Pineal gland
B. Thymus
C. Hypothalamus
D. Pancreas
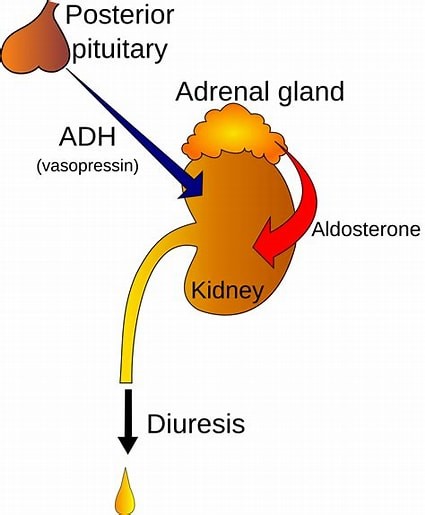
The Correct Answer is C.The hypothalamus is a region of the brain that synthesizes antidiuretic hormone (ADH), also known as vasopressin.
ADH is then transported to the posterior pituitary gland via neurohypophysial capillaries, where it is stored until it is ready to be secreted into the circulation.
Choice A.
Pineal gland is not correct because it is a small endocrine gland located in the brain that secretes the hormone melatonin, which regulates sleep-wake cycles, but it does not synthesize ADH.
Choice B.
Thymus is not correct because it is a gland located in the chest that produces hormones involved in immune system development, but it does not synthesize ADH.
Choice D.
Pancreas is not correct because it is a gland located behind the stomach that secretes hormones such as insulin and glucagon, which regulate blood sugar levels, but it does not synthesize ADH.
Question 8:
In a hypertonic solution, water flows through aquaporins embedded in the plasma membrane of the cell.
This type of transport is best known as which of the following?
A. Facilitated diffusion
B. Active transport
C. Osmosis
D. Diffusion
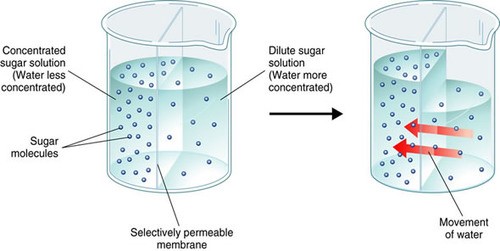
The Correct Answer is C.Osmosis is the movement of water molecules across a selectively permeable membrane from an area of higher water concentration to an area of lower water concentration.
In a hypertonic solution, the concentration of solutes outside the cell is higher than inside the cell, so water flows out of the cell through aquaporins embedded in the plasma membrane to balance the concentration gradient.
Choice A.
Facilitated diffusion is not correct because it is a type of passive transport that involves the movement of molecules across a membrane through specific transport proteins, but it does not specifically refer to the movement of water molecules.
Choice B.
Active transport is not correct because it is a type of transport that involves the movement of molecules against their concentration gradient and requires energy in the form of ATP, but osmosis is a passive process that does not require energy.
Choice D.
Diffusion is not correct because it refers to the movement of molecules from an area of higher concentration to an area of lower concentration, but it does not specifically refer to the movement of water molecules.
Question 9:
Which of the following is the structure through which blood exits the glomerulus?
A. Efferent arteriole
B. Proximal tubule
C. Distal tubule
D. Afferent arteriole
The Correct Answer is A.The glomerulus is the main filtering unit of the kidney.
It is formed by a network of small blood vessels (capillaries) enclosed within a sac called the Bowman’s capsule.
The blood supply to the glomerulus is provided via the afferent arteriole.
The blood then flows through the capillary network, where it gets filtered, and then leaves the glomerulus via the efferent arteriole.
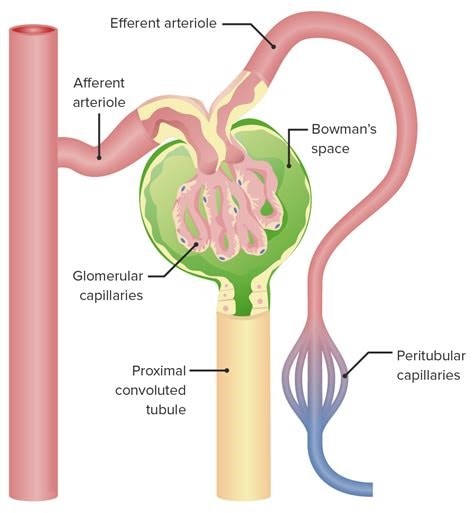
Choice B.
Proximal tubule is not correct because it is where the ultrafiltrate collected in the Bowman’s space drains directly into.
Choice C.
Distal tubule is not correct because it is not mentioned in relation to blood exiting the glomerulus.
Choice D.
Afferent arteriole is not correct because it provides blood supply to the glomerulus.
Question 10:
Which of the following summarizes a change that takes place as a solid turns to a liquid?
A. Particles become less ordered.
B. Particles have a decrease in mobility.
C. Particles move closer together
D. Intermolecular forces between particles become stronger.
The Correct Answer is A.As a solid turns to a liquid, the particles become less ordered and more free to move around.
Choice B is not correct because particles have an increase in mobility as a solid turns to a liquid.
Choice C is not correct because particles move further apart as a solid turns to a liquid.
Choice D is not correct because intermolecular forces between particles become weaker as a solid turns to a liquid.