Which of the following best describes the result of using a catalyst in a chemical reaction?
A more desirable product is often formed.
The reaction is completed in a shorter amount of time.
A greater amount of heat energy is released by the reaction.
The yield of product is increased.
Answer and Rationale
The Correct Answer is B.
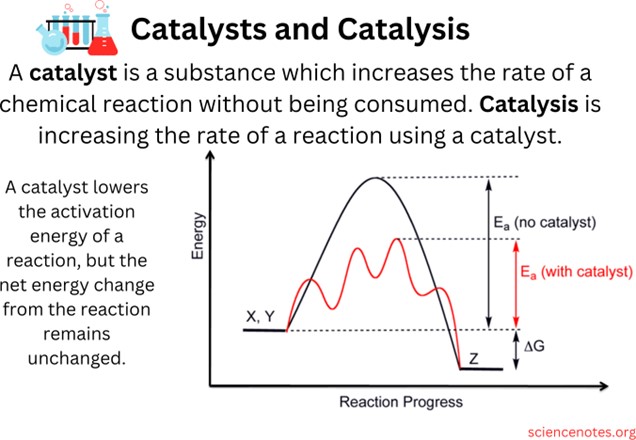
The result of using a catalyst in a chemical reaction is that the reaction is completed in a shorter amount of time. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. A catalyst provides an alternative pathway for the reaction, one that has a lower activation energy than the uncatalyzed pathway.
The other options are not correct because they do not accurately describe the result of using a catalyst in a chemical reaction. A more desirable product is not necessarily formed, a greater amount of heat energy is not necessarily released by the reaction, and the yield of the product is not necessarily increased as a result of using a catalyst.
Acess all questions on ExamGateway's Free Nursing TestBank