Introduction to Acids and Bases
I. Introduction to Acids and Bases
Acids and bases are two fundamental categories of chemical substances that play crucial roles in various chemical reactions and natural processes. Understanding their properties and behavior is essential in chemistry and has significant implications in daily life.
A. Definition of Acids:
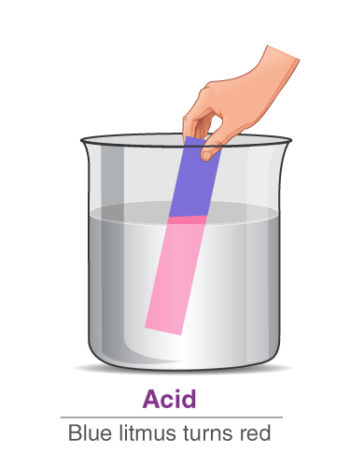
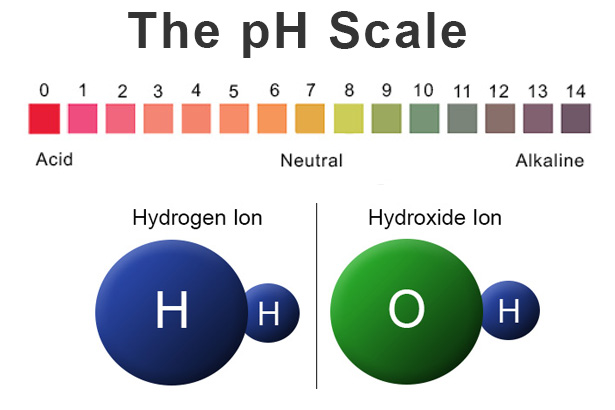
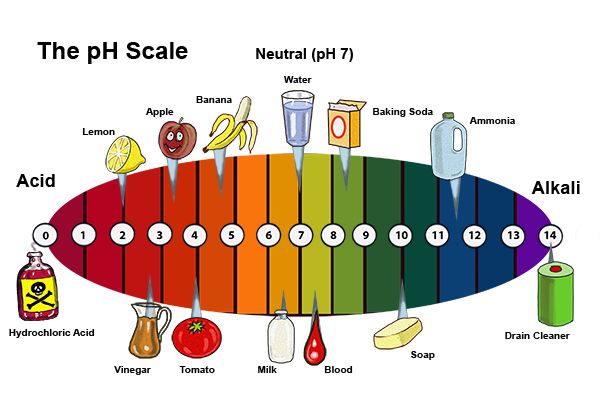
Acids are substances that donate protons (H⁺ ions) in aqueous solutions. They are characterized by their sour taste, ability to turn blue litmus paper red, and their corrosive properties. Acids react with metals to produce hydrogen gas and with bases to form salts and water. Some common examples of acids include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and citric acid (found in citrus fruits).
B. Definition of Bases:
Bases, also known as alkalis, are substances that accept protons (H⁺ ions) or donate hydroxide ions (OH⁻) in aqueous solutions. They typically have a bitter taste, feel slippery to the touch, and turn red litmus paper blue. Bases react with acids to form salts and water. Common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH₃).
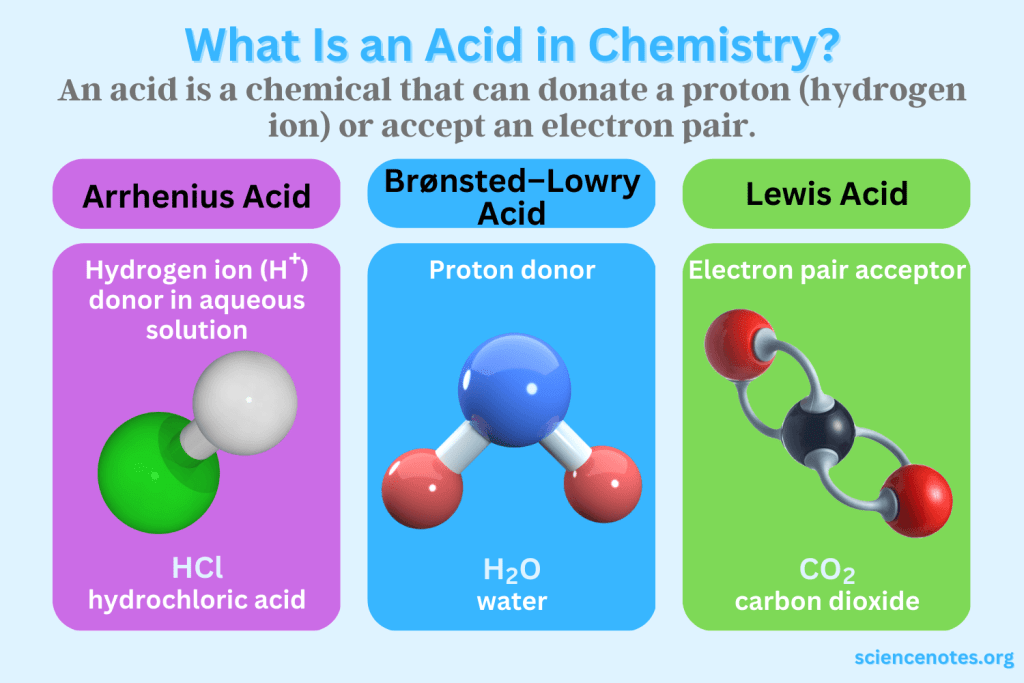
C. Historical Perspective:
The study of acids and bases dates back to ancient times, with early civilizations recognizing their properties in various natural substances. However, the systematic study of acids and bases began in the late 17th and early 18th centuries with the works of notable scientists such as Robert Boyle, Antoine Lavoisier, and Humphry Davy. Over time, different theories and models have been developed to explain the behavior of acids and bases, leading to our modern understanding of these substances.
D. Importance in Chemistry and Daily Life:
Acids and bases play vital roles in numerous chemical reactions, including neutralization reactions, precipitation reactions, and acid-base equilibria. They are widely used in industrial processes, such as the production of fertilizers, pharmaceuticals, and cleaning agents. Additionally, acids and bases are essential in biological systems, influencing processes like digestion, cellular metabolism, and enzyme activity. In daily life, they are present in various household products, ranging from food and beverages to cleaning solutions and personal care items.
Understanding the properties and behavior of acids and bases is foundational to the study of chemistry and is essential for addressing practical challenges in diverse fields, making them subjects of significant scientific interest and practical relevance.