Properties of Bases
Bases are substances that can accept protons or donate pairs of electrons. They exhibit several characteristic properties:
A. Bitter Taste
Bases have a bitter taste, which is distinct from the sour taste of acids. However, this property is not often used to identify bases due to safety concerns, as many common bases are caustic and can be harmful if ingested.
B. Effect on Indicators
Bases can change the color of certain indicators, providing a simple way to detect their presence. For example:
- Litmus paper turns blue in the presence of a base, indicating a shift from the acidic to the basic form.
- Phenolphthalein, a commonly used indicator, changes from colorless to pink in basic solutions with a pH greater than approximately 8.2.
- Methyl orange transitions from red in acidic solutions to yellow in basic solutions.
C. Reaction with Acids
Bases neutralize acids through a chemical reaction known as neutralization, where a salt and water are formed. The general reaction between a base (B) and an acid (HA) is represented by the equation:
\[ B + HA \rightarrow BA + H_2O \]
Here, BA represents the salt formed by the cation of the base and the anion of the acid.
D. Reaction with Metals
Bases can react with certain metals to form salts and release hydrogen gas. However, this reaction is not as common or vigorous as the reaction of acids with metals. The general reaction can be represented as follows, taking the reaction between sodium hydroxide (NaOH) and aluminum (Al) as an example:
\[ 2NaOH + 2Al \rightarrow 2NaAlO_2 + H_2 \]
E. Conductivity
When dissolved in water, bases ionize to produce hydroxide ions (\(OH^-\)), which can conduct electricity. However, they typically exhibit lower conductivity compared to strong acids. The conductivity of a base solution depends on the concentration of hydroxide ions (\(OH^-\)) present.
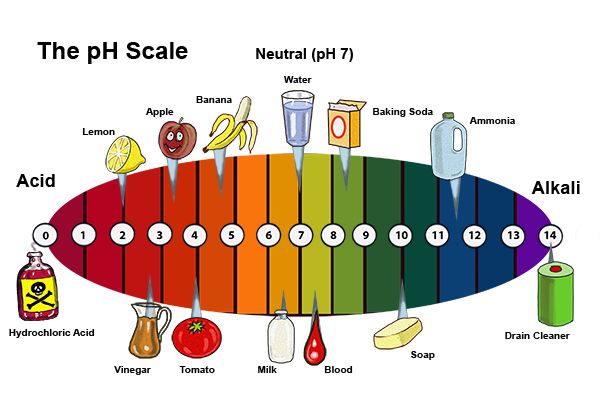
F. pH Scale and Alkalinity
Bases have pH values greater than 7 on the pH scale, indicating their alkaline nature. The higher the concentration of hydroxide ions (\(OH^-\)), the more alkaline the solution, and the higher the pH. The pH of a basic solution can be calculated using the expression:
\[ \text{pH} = -\log[\text{OH}^-] \]
Where \( [\text{OH}^-] \) represents the concentration of hydroxide ions in moles per liter.
Understanding these properties is crucial for identifying and working with bases in various chemical reactions and applications.
More on AmplifyGlobe
Introduction to Acids and Bases
 Introduction to Acids and Bases: Definition of Acids, Definition of Bases, Histo Read More
Introduction to Acids and Bases: Definition of Acids, Definition of Bases, Histo Read More
Properties of Acids
 Acids exhibit several characteristic properties that distinguish them from other Read More
Acids exhibit several characteristic properties that distinguish them from other Read More
Strength of Acids and Bases
 The strength of an acid or base refers to its ability to dissociate into ions in Read More
The strength of an acid or base refers to its ability to dissociate into ions in Read More